Treatment
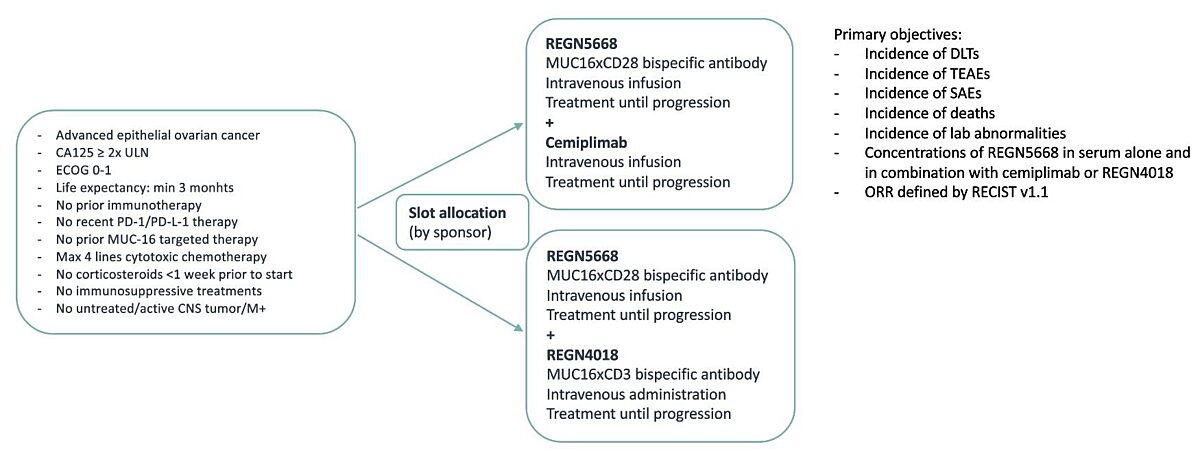
This trial consists of two different treatment options:
- REGN5668 + Cemiplimab
- REGN5668 + RENG4018
REGN5668 is a MUC16xCD28 bispecific antibody. REGN4018 is a MUC16xCD3 bispecific antibody.
REGN5668, REGN4018 and Cemiplimab are administered intravenously.
Patients are assigned to one of either group based on their disease characteristics and the availability of slots.
No randomisation will be performed
Treatment duration
The treatment can continue until disease progression, unacceptable toxicity or withdrawal of consent
Eligibility
Main inclusion criteria:
- Diagnosis of advanced epithelial ovarian cancer (except carcinosarcoma), primary peritoneal, or fallopian tube cancer
- At least 1 line of platinum-based systemic therapy
- CA-125 level ≥2x ULN (in screening)
- (ECOG performance status of 0 or 1
- Has a life expectancy of at least 3 months
Main exclusion criteria:
- Prior anti-cancer immunotherapy
- Recent treatment with anti-Programmed Cell Death (PD-1)/PDL-1 therapy
- Another malignancy within the last 5 years that is progressing, requires active treatment, or has a high likelihood of recurrence as defined in the protocol
- Prior treatment with a MUC16-targeted therapy
- More than 4 lines of cytotoxic chemotherapy (including antibody drug conjugates)
- Requiring ongoing/continuous corticosteroid therapy as defined in the protocol within 1 week prior to the first dose of study drug
- Has ongoing or recent (within 5 years) evidence of significant autoimmune disease that required treatment with systemic immunosuppresive treatments as defined in the protocol
- Has untreated or active primary brain tumor, CNS metastases, leptomeningeal disease, or spinal cord compression as defined in the protocol
Disclaimer: This is an overview of the main in- and exclusion criteria which can also be found on clinicaltrials.gov (NCT04590326). Criteria might change during the course of the study due to amendments. Inclusion in the trial remains the responsibility of the principal investigator of the trial and will depend on patient eligibility and slot availability.
Contact
In case you feel your patient might be eligible for this trial or in case you want to have more information about this trial, please feel free to contact us via: lgog@uzleuven.be